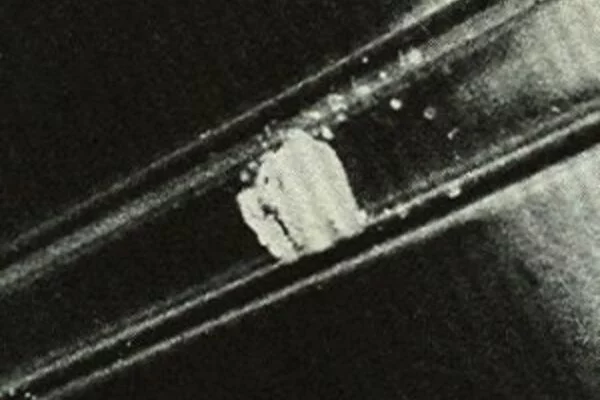
Californium(III) chloride is an inorganic compound with a chemical formula CfCl3. As in californium(III) oxide (Cf2O3) and other californium halides, including californium(III) fluoride (CfF3) and iodide (CfI3), the californium atom has an oxidation state of 3.
Preparation
Californium(III) chloride can prepared by reacting californium(III) oxide with hydrogen chloride.
- Cf2O3 6 HCl → 2 CfCl3 3 H2O
Properties
Chemical properties
When heating californium(III) chloride until 500 °C, it can hydrolyse to produce californium oxychloride.
Physical properties
Californium(III) chloride is soluble in water, giving Cf3 and Cl− ions. This salt has an emerald-green color. Its crystal structure is hexagonal. It is strongly radioactive.
See also
- Californium
- Californium compounds
References